QBank Newsletter (Volume 1, Issue 1)
Hello!
We are happy to announce our first-ever QBank newsletter! In this issue we have covered some of the recent updates that have happened within and beyond the world of Qbank.
In this newsletter, the focus is entirely on COVID-19 related updates.
1, 6 new symptoms added:
Recently, the Centers for Disease Control & Prevention (CDC) has added 6 more symptoms to aid the diagnosis of COVID- 19. These are:
- Chills
- Muscle pain
- Headache
- New Loss of taste or smell
- Sore Throat
- Diarrhoea
Source: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
2, Kavach ELISA
The Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), Pune have developed and validated the indigenous IgG ELISA kit called KAVACH ELISA for antibody detection of COVID-19. This is an important surveillance tool to understand the proportion of the infected population.
Source:https://main.icmr.nic.in/sites/default/files/press_realease_files/ICMR_PressRelease_14052020.pd
3, Updated Guidelines on COVID-19 Sample Collection and Testing
The Ministry of Health and Family Welfare (MoHFW) has updated guidelines on sample collection and testing of COVID-19.
- Samples: Nasopharyngeal and Oropharyngeal swabs
- Swab material: Dacron or polyester flocked swabs
- Note: Calcium alginate swabs or swabs with wooden shafts are not used, as they may contain substances that inactivate the virus and inhibit PCR testing.
- Transport to Laboratory at 4 °C
- Storage till testing: ≤5 days at 4 °C or >5 days at -70 °C
For the transportation of samples VTM (viral transport medium) containing antifungal and antibiotic supplements is to be used. Repeated freezing and thawing of specimens should be avoided.
The nasopharyngeal and oropharyngeal swabs should be placed in the same tube to increase the viral load.
4, 5th version of Clinical Management Protocol
The Ministry of Health and Family Welfare (MoHFW), recently, has issued the fifth version of the clinical management protocol for COVID 19. This has been tabulated as follows:
| MILD | MODERATE | SEVERE |
|---|---|---|
| Fever URTI | Pneumonia with no signs of severe disease (RR ≥ 24 SpO2 <94%) | Respiratory distress (RR ≥ 30 SpO2 <90%) |
| Admit to COVID Centre/Home Quarantine | Admit to Dedicated COVID Health Centre | Admit to Dedicated COVID Hospital |
| Precaution and Hygiene with Home quarantine Symptomatic Management Tab HCQ 400mg BD x1 Day Followed by 400mg OD x4 Days (in high-risk case*) Watch for warning signs and seek medical attention if needed | Oxygen: Target 92-96 % SpO2 Daily monitoring (CBC and RFT, LFT, Absolute lymphocyte count, ECG ) 48 hourly Monitoring (CRP, D- dimer & Ferritin) Tab HCQ 400mg BD x1 Day Followed by 400mg OD x4 Days Anticoagulation (UFH or LMWH) Enoxaparin 40mg SC OD IV Methylprednisolone 0.5 to 1mg for 3- 5 days Investigational therapies Inj Remdesivir 200mg on Day 1 followed by 100mg for next 4 days Convalescent Plasma 200ml/day for every 24 hours | CPAP or NIV Intubation if needed Prone ventilation considered in refractory hypoxemia IV Methylprednisolone 1 to 2mg/kg/day for 7 days High prophylactic dose of Anticoagulation (UFH or LMWH) Enoxaparin 40mg SC BD Sepsis/ Septic Shock: managed according to their protocol Sedation or Nutrition therapy Investigational therapies Inj Tocilizumab 8mg/kg one dose, repeated after 12 to 24 hour if no improvement occurs with the first dose. |
Source: COVID 19 Management Protocol by MOHFW version 5 (03.07.2020)
https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf]
5, New Treatment Modalities
The MoHFW has approved the following for treating COVID 19 patients:
- Remdesivir, an RNA polymerase inhibitor (Emergency Use Authorization issued by FDA ) may be considered in adults and pediatric patients with the moderate-severe disease who require supplemental oxygen.
Note: Co-administration of Remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in the reduced antiviral activity of Remdesivir. - Off label use of convalescent plasma and Tocilizumab may be considered in patients with moderate disease who are not improving despite the use of steroids and oxygen requirement is progressively increasing.
Note: The use of convalescent plasma therapy for the treatment of patients with Covid-19 is under open-label, randomized, controlled Phase II trial conducted by the ICMR. - Hydroxychloroquine is to be used early in the disease in high-risk populations.
- Glucocorticoids can be used for a short period of time (3 to 5 days) in patients whose condition is deteriorating progressively. This is depicted by worsening oxygen indicators, imaging, and excessive activation of the body’s inflammatory response.
- Favipiravir, the viral polymerase inhibitor has been approved in tablet form for mild to moderate COVID-19 infection.
Sources:
1, https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf
2, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
3, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-newly-discovered-potential-drug-interaction-may-reduce
MCQ IDs of the updated MCQs in QBank:
Newly Added MCQs: MB3386 | MD0964 | MD3188 | MD3189 | MC4159 | MD3187 | MA8142 | MA6731 | MA7567 | MD3379 | MA6361 | Pearl No. 2444
You can copy the MCQ IDs and search for it on your App
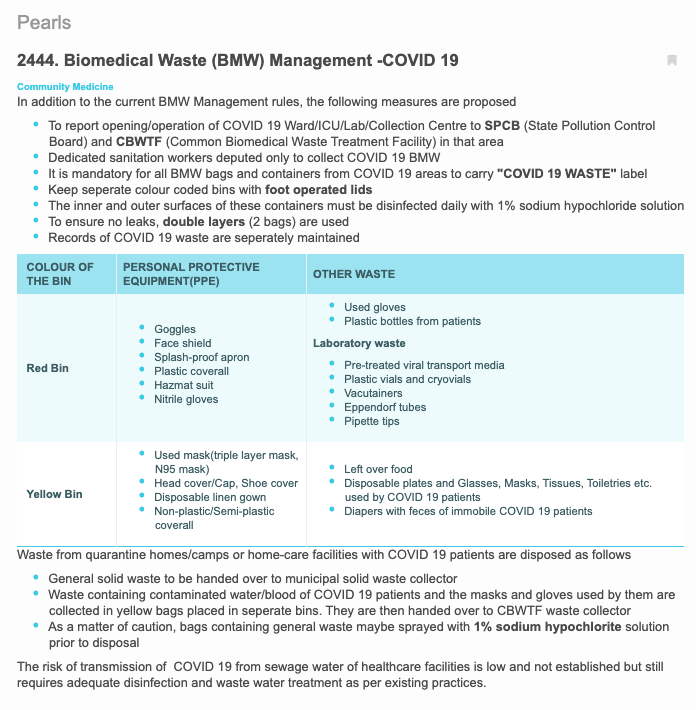
Preview of Pearl No. 2444:


Nice
Is HCQ useful in treating Covid -19 patients?
Kindly provide this information in app only
Kindly post this in App only tough to read and understand
Useful
Where should i subscribe for email notifications of marrow newsletters.?