QBank Fortnightly Newsletter (VOLUME: 1, ISSUE: 6)
Updates from Robbins & Cotran latest edition and MOHFW.
Hello!
We are back with the next issue of our newsletter. As always, we have brought you a few more new and updated MCQs (and pearls) from the world of Qbank.
In this issue, we talk about some fascinating topics, from rabies vaccination to ninja stars!
Have a look.
1. Rabies vaccination:
MCQ IDs: MD0180, MA7901, MB8304, MC7168. Pearl id: 1725.
Pre-exposure prophylaxis:
- Pre-exposure rabies vaccination consists of three intramuscular (IM) or intradermal (ID) doses given on days 0, 7, and 21 or 28.
- For adults, the vaccine should always be administered in the deltoid region of the arm; for children less than one year of age, the anterolateral area of the thigh is recommended.
Note: Rabies vaccine should never be administered in the gluteal area. Administration in this manner will result in lower neutralizing antibody titers.
- A booster is recommended if rabies virus neutralizing antibody titers have dropped to less than 0.5 IU/ml.
Post-exposure prophylaxis:
Intramuscular regimen:
- The five-dose (Essen) regimen (1-1-1-1-1) is administered on days 0, 3, 7, 14, and 28, IM, into the deltoid muscle (adults) or anterolateral part of the thigh (children).
Intradermal regimen:
- The Updated Thai Red Cross Regimen (2-2-2-0-2) is administered on days 0, 3, 7, and 28, ID, on two sites (one on each deltoid area).
Rabies Immunoglobulin (RIG):
RIG should be administered to all category three III exposures (along with category II and III exposures in immunocompromised individuals).
Note: For exposed or re-exposed patients who can document previous complete PrEP or PEP, the following guidelines would be applicable:
- Proper wound management should be done.
- There is no need for the administration of RIG.
- One site ID or IM vaccine administration on days 0 and 3.
Only adequate wound washing would be required in case of re-exposure where the animal bite victim has documented proof of complete PEP or PrEP within the last three months.
People who have previously received full post-exposure treatment with neural tissue vaccines (NTV) or vaccine of unproven potency or cannot document complete PEP or PrEP treatment should be treated as a new case and given full PEP.
Last updated on October 2nd, 2020.
Reference:https://ncdc.gov.in/WriteReadData/linkimages/GuidelinesforRabiesProphylaxis.pdf
2. Prevention of vertical transmission of Hepatitis B Virus (HBV) (MCQ id: MC0967)
Elimination of HBV infection as a public health threat requires a reduction in the prevalence of hepatitis B surface antigen (HBsAg) to below 0.1% in children five years of age. To achieve this goal, WHO has recently issued guidelines on antiviral prophylaxis in pregnancy to prevent mother-to-child transmission of HBV.
- Tenofovir prophylaxis should be started in all pregnant women who are testing positive for HBV infection (HBsAg positive) with an HBV DNA ≥ 200,000 IU/mL (≥ 5.3 log10 IU/mL) from the 28th week of pregnancy until birth. This should be in addition to three-dose hepatitis B vaccination in all infants, including a timely birth dose.
- HBeAg testing should be used where HBV DNA testing is not available, to determine treatment eligibility for tenofovir prophylaxis to prevent mother-to-child transmission of HBV.
Reference: https://apps.who.int/iris/bitstream/handle/10665/333391/9789240002708-eng.pdf?sequence=1&isAllowed=y
3. Efferocytosis (MCQ id- MD5037)
Efferocytosis is a new term introduced in the Robbins and Cotran 10th edition. It is a specialized type of phagocytosis of apoptotic bodies. Here, various ligands bind to the apoptotic bodies and generate an ‘eat me’ signal. These signals are recognized by the receptors of phagocytes, which bind and engulf the bodies.
It is carried out mainly by conventional phagocytes like macrophages and dendritic cells. However, many fibroblast and epithelial cells can also take part in efferocytosis.
Reference- Robbins and Cotran 10th edition, page no 46
4. Kruppel like factor 2 (KLF 2) and atherosclerosis (MCQ- MD5038)
According to the 10th edition of Robbins and Cotran, production of transcription factors particularly Kruppel like factor 2 (KLF 2) is increased by laminar non-turbulent blood flow. KLF 2, in turn, activates the atheroprotective gene and inactivates the inflammatory gene transcription. This suggests that elevating KLF2 expression may be a novel strategy for the prevention and treatment of atherosclerosis.
Reference -Robbins and Cotran 10th edition, page no 498
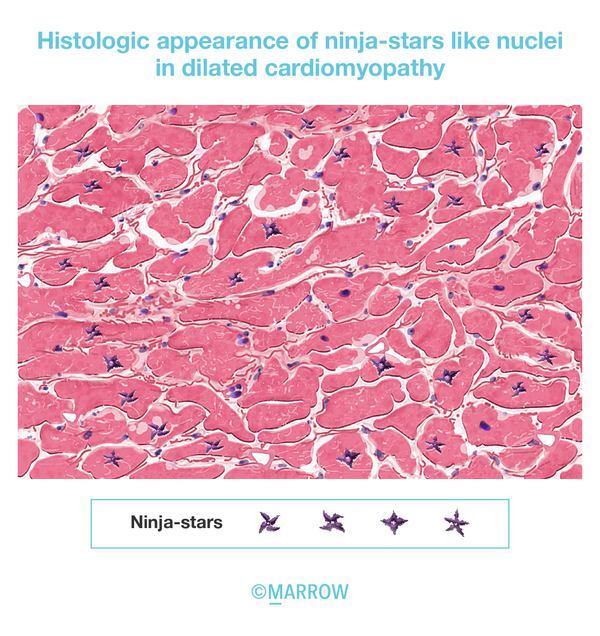
5. Ninja-star appearance (MCQ id- MD5039)
As per the recent edition of Robbins and Cotran. In most patients with dilated cardiomyopathy caused by Titin truncating mutation show the histologic appearance like ‘ninja-star’- an enlarged, bizarre, hyperchromatic nuclei.
Reference- Robbins and Cotran 10th edition, Page no 571
——————————————————————————————————————–
Click here to check out the previous issues of newsletters.


How can i acces the earlier newsletters from marrow??
Open the “blog” option on this page on the top left corner and from there you can access all the newsletters.
Always Thank you Marrow….
✌🏻
Wow….thnx marrow 😃
Thank you so much Marrow
Thanks
Thanksalot marrow😊
Thank you marrow
Thank you!
Thank you marrow😇
The newsletter is a very impressive initiative, it is a way more superior and neat approach to inculcate information to the readers rather than the messy bombardment of information. Thanks marrow
Thank you marrow 🤗
Thanks marrow
Thanx marrow👏👏👏
Thank you Marrow
Thank you so much Marrow..
Marrow is the best…. Really thankful to marrow for taking care of thousands and lakhs of student…
Thank you marrow for regular updates
Very informative 👏
Ahaha I love the ninja stars for reference! They do look remarkably similar. Thanks for the update.
Thnk u 😇 marrow
What an initiative extremely this has an edge over with the other online platforms available in the market that’s why marrow s always stand one step ahead of them in all the things …kudos to the marrow team
Thanks for the update Marrow
How can i revisit these newsletter when I want to revise something?
Thank you marrow for keeping us updated..
Ty marrow.
Tq
Nice data
Thank you so much, Marrow. Quite a way of presentation!
How to access the previous issues of news letters?? And the video by PSM faculty on latest TB guidelines
Thank you so much for the update @Marrow. A suggestion, Please give notification on the app on the day when the issue is published. It was published on sep 18 and today is 21!!
Thanks marrow 😃
Thank you marrow
Thanks
Thank u marrow team for the latest updates
Thank you marrow for this latest update
Awesome
Thanks
Thank you very much
Thank you Team Marrow:)
Thank you. plz share information timely,It shows responsibility
Thanx a ton
thank u
Thank you marrow
Thanks a lot
Thank u marrow
Great initiative! Very systematic for learning. Keep coming up with all new, different and helpful features!!
Thankyou marrow
Thank you . Keep updating
Thank u marrow
Thank you marrow for the new updates
How to get the old fortnight newsletters?
Thanks for updating
Thank you marrow for the updates..
This is really appreciable initiative. Thankyou Marrow.
Thank u team marrow ,, for always be ter,,,
Thank u team marrow ,, for always be ter,,,
Thank you marrow
Thank yu team marrow 😇
Superb initiative 🙌🙌🙌
Thankyou marrow 🧡
Thank you 🙂
Marrow as awesome as always…. thank u✌️🤩
Thank you for marrow team
Waiting for more updates in classifications and PSM updates
How to find old newsletters?… please anyone help me
How do i get all d updates .. i missed d previous vol .. pls help marrow team
Thank you for the updates! 👍
Thank you
Does anyone know how to get the previous letters.
Thanks marrow ❤️
We like u
People who’ve formerly acquired complete post-publicity remedies with neural tissue vaccines (NTV) or vaccines of unproven efficiency or can not record entire PEP or PrEP remedy need to be dealt with as a brand new case and given complete PEP. thanks for sharing this blog